FDA approvals data
8 min

EXPERT INSIGHT
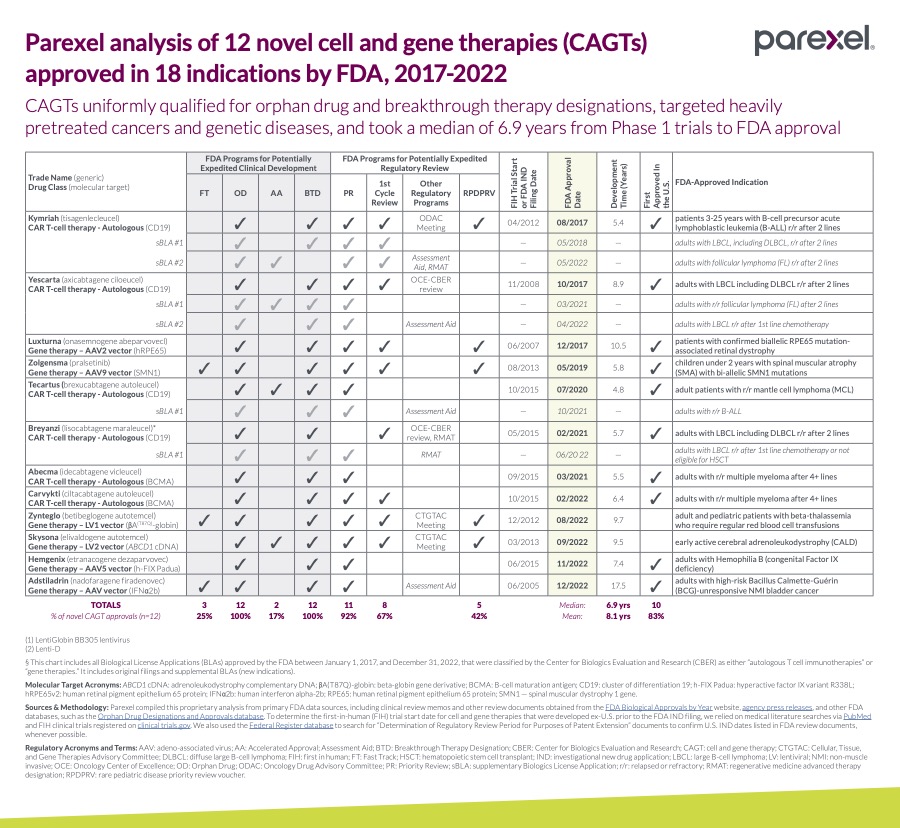
Parexel analysis of 12 novel cell and gene therapies (CAGT) approved in 18 indications by FDA, 2017 - 2022
From January 1, 2017, to December 31, 2022, the FDA approved 12 novel cell and gene therapies. Below is a Parexel analysis based on primary FDA data sources.1
Modalities and development times
50% percent (6 of 12) therapies were CAR T Cell Therapies. The overall development time—from IND or FIH —to market, took an average of 5.4 Years. (Median: 4.8 | Range: 4.2 to 8.4)
50% percent (6 of 12) therapies were Gene Therapies. The overall development time—from IND or FIH—to market, took an average of 10.1 Years. (Median: 9.6 | Range: 5.8 to 17.5)
Key to Acronyms:
IND: investigational new drug application; FIH: first-in-human clinical trial.
50%
CAR-T Cell Therapies
5.4years
The overall development time—from IND or FIH—to market, took an average of...
50%
Gene Therapies
10.1years
Therapeutic areas and indications
42% percent (5 of 12) therapies treat Genetic Diseases:
- Retinal Dystrophy
- Spinal Muscular Atrophy
- Beta-Thalassemia
- Cerebral Adrenoleukodystrophy
- Hemophilia B
58% percent (7 of 12) therapies treat Cancers
- Leukemia B-ALL (3rd Line in heavily pretreated patients)
- Lymphoma LBCL (3rd Line in heavily pretreated patients)
- Lymphoma MCL (2nd Line in heavily pretreated patients)
- Lymphoma LBCL (3rd Line in heavily pretreated patients)
- Multiple Myeloma (5th Line in heavily pretreated patients)
- Multiple Myeloma (5th Line in heavily pretreated patients)
- Bladder Cancer (1st Line, High-risk in heavily pretreated patients)
42%
Treat Genetic Diseases
58%
Treat Cancers
FDA expedited development programs
100% percent (12 of 12) therapies used Orphan Drug development programs.
100% percent (12 of 12) therapies used Breakthrough Therapy development programs.
25% percent (3 of 12) therapies used Fast Track development programs.
17% percent (2 of 12) therapies used Accelerated Approval development programs.
8% percent (1 of 12) therapies used RMAT development programs.
100%
Orphan Drug Development Programs
100%
Breakthrough Therapy Development Programs
25%
Fast Track Development Programs
17%
Accelerated Approval Development Programs
8%
RMAT Development Programs
Other FDA metrics
83% percent (10 of 12) first approved in the United States.
67% percent (8 of 12) first cycle review.
50% percent (6 of 12) risk evaluation and mitigation strategy (REMS).
25% percent (3 of 12) FDA advisory committee meeting.
25% percent (3 of 12) clinical hold during development.
83%
First Approved in The United States
67%
First Cycle Review
50%
Risk Evaluation & Mitigation Strategy (REMS)
25%
FDA Advisory Committee Meeting
25%
Clinical Hold During Development
Year by year FDA approvals of novel CAGT products
- 2017 saw three approvals (two CAR-T cell therapies and one gene therapy-Luxturna).
- 2018 saw zero.
- 2019 saw one (gene therapy).
- 2020 saw one (CAR-T cell therapy).
- 2021 saw two (both CAR-T cell therapies).
- 2022 saw five (one CAR-T cell therapy-Carvykti and four gene therapies).
25%
2017Kymriah, Luxturna, Yescarta
0%
2018
8%
2019Zolgensma
8%
2020Tecartus
17%
2021Abecma, Breyanzi
42%
2020Adstiladrin, Carvykti, Hemgenix, Skysona, Zyteglo
Data Sources and Methodology
1. Parexel analysis based on primary FDA data sources, including clinical review memos and other review documents obtained from the FDA Biological Approvals by Year website, agency press releases, and other FDA databases, such as the Orphan Drug Designations and Approvals database. We analyzed all biologics license applications (BLAs) approved by the FDA’s Center for Biologics Evaluation and Research (CBER) from January 1, 2017, to December 31, 2022, that were classified as either “autologous T cell immunotherapies” or “gene therapies.” Totals reflect approvals for new molecular entities (NMEs) (original BLAs) and exclude supplemental BLAs (new indications).